|
Cooling Tower Water Quality
Consider the cooling tower, along with the other
mechanical components, as the cooling system hardware, while the
water flowing through the cooling tower is the system software.
Cooling towers help regulate temperature by rejecting heat from
air-conditioning systems or by cooling hot equipment. In doing
so, they use significant amounts of water. The thermal efficiency,
proper operation and longevity of the water cooling system all
depend on the quality of water and its reuse potential.
In a cooling tower, water is lost through evaporation,
bleed-off, and drift. To replace the lost water and maintain its
cooling function, more make-up water must be added to the tower
system. Sometimes water used for other equipment within a facility
can be recycled and reused for cooling tower make-up with little
or no pre-treatment, including the following
Water condition is not often thought of as a potential
variable over time. The cooling water essentially is invisible
to the operator, but when left unattended, water supports biological
growth, and corrodes or scales equipment. Proper ongoing treatment
is important to process operation and efficiency.
Cooling loops remove waste heat from the process
and function by allowing water to evaporate to the atmosphere.
The evaporated water must be replaced continuously by fresh make-up
water.
Without a bleed stream, commonly known as blow-
down, the dissolved solids in the original system’s water
volume, plus dissolved solids added by the make-up water, will
accumulate in the system until precipitation begins. Eventually
an unbled system will fill with solid scale material.
To balance the incoming water solids, a small
portion of the circulating water stream with its elevated solids
level is removed to drain. An equilibrium is then established
between the added water replacing evaporation and high solids
water losses, drift and blow-down.
The term cycles of concentration is defined as
the
resulting ratio of concentrated solids in the
circulating water compared to that in the fresh make-up. Calculation
of cycles is performed using the equation below. Alternatively,
the blow-down required to establish a given equilibrium-cycles
level can be derived from this equation:
C = (E+D+B)/(D+B) where:
C = Cycles of concentration.
E = Evaporation, approximately gpm* x °F
range x 0.0008.
D = Drift loss, approximately gpm x 0.0002.
B = Blow-down, gpm. *Gallons per minute |
 |
|
|
It is the open nature of these systems that determines
the unique water problems that they exhibit. Cooling towers concentrate
solids and the air contact in these open systems allows the build-up
of contaminants, organics, bacteria and their food sources in
the circulating cooling water.
These unique evaporative water system problems
– concentration and air washing – must be dealt with
or process disasters will follow. Water is both the static and
dynamic basis for controlling such problems.
Poor water maintenance will create certain and
predictable problems in open cooling loops. These problems are
detailed below:
Scale formation: Scale formation is the creation
of a precipitated solid. By coating heat-exchange surfaces, this
�Scale thickens the barrier to heat trans- fer,
thus reducing the efficiency of the cooling system. solid material
interferes with efficiency of the system’s heat- exchange
surface and also blocks water flow at the cooling tower’s
basin or fill. Cooling loop precipitates generally are calcium
carbonate crystals. In a few cases, these are calcium sulfate
or silica solids.
Scaling occurs because specific dissolved solids,
in the case of calcium carbonate scale, namely calcium and bicarbonate
alkalinity, have exceeded their solubility limits and are forming
solids. Calcium carbonate crystals commonly precipitate on critical
surfaces, like the heat exchanger tube interior walls. The scale
thickens the barrier to heat transfer, thus reducing the efficiency
of the cooling system. Severe scaling is shown in the photo above.
Corrosion: Corrosion is the process of metal dissolution,
usually by oxidation, resulting in substantial material breakdown
and premature degradation of system equipment. The oxidation process,
in a very simplified form, is the movement of electrons from metal
system components into the water medium provided in wet systems
and subsequently to a corrosion product of substantially different
form than the original base material.
This process degrades the metal, reduces its strength,
thickness, and in some extreme cases, creates pits and then holes
in the material. At some point in the corrosion process, the metal
can no longer do its job as a system component. Corrosion, in
general, and pitting corrosion, in particular, must be guarded
against in order to ensure the long- term integrity of the cooling
system. Extensive corrosion is shown in the photo here.
Corrosion, in general, and pitting corro- sion,
in particular, must be guarded against in order to ensure the
long-term integrity of the cooling system. Biological fouling:
Water left unattended for any significant length of time will
grow bacteria, fungi, algae and even protozoa. This diversity
of growth, allowing organisms to flourish in a protected environment,
results in
well-established and difficult-to-remove biofilm.
This growth process and its attachment to system surfaces is called
biological fouling.
This growth is considered by many to be the root
of most cooling-loop water treatment problems. Those problems
include heat transfer efficiency reduction, cooling tower fill
fouling, water-flow blockages, microbiologically induced corrosion
and human health concerns.
All of these effects are dramatic and serious.
Biological control must, therefore, be a primary part of good
water system control. A picture of a biologically fouled cooling
tower fill is shown here.
Biological fouling is the root of most cooling-loop
water treat- ment problems, according to many experts. Water management
strategies
There are a number of strategies for combating
each of the problems described above. One must evaluate the system
components, the available water and water management techniques
available for a given site to determine the best solution for
a specific case. Below are discussions of the control techniques
for each problem area.
Preventing scale: To prevent buildup of mineral
scales, the first and most important choice is the operating water
chemistry of the cooling loop as determined by the inlet and outlet
water volumes from the system. An operator must choose this operating
cycles level, using the equation cited earlier in this article,
before any subsequent treatment choices can be made.
Practical tools for automating water removal from
the system are an instrument to measure circulating water conductivity,
solids in the water and a signaled solenoid valve to bleed the
water system when necessary. The conductivity set point is the
most critical factor in operating an open evaporative cooling
loop.
Once the type of solids and their concentrations
are known, an effective inhibitor selection can be made to ensure
that deposition does not occur on vital heat- exchange surfaces
in the process equipment. One can inhibit the most common scales
by using sequestering agents like HEDP (HydroxyEthylidene 1, 1
DiPhosphonic acid) and/or polymers like polyacrylate.
�Preventing corrosion: Corrosion is prevented
by a number of system choices and ongoing prevention strategies.
First, the bulk water chemistry at operating cycles of concentration
as determined in the scaling section will dramatically impact
the corrosion rates in a cooling system.
Once the system chemistry is known, one can then
carefully choose the system materials for the environment and
for compatibility with one another. Third, it is essential to
keep the system clean. Deposits provide locations where bacteria
can act to create nonstandard water conditions that dramatically
increase the local corrosion rate.
Therefore, the system must be substantially free
from biological activity and largely clear of debris on surfaces,
particularly in areas where suspended solids will settle. Continuous
filtration or regular and thorough maintenance cleanings will
accomplish this.
Finally, use corrosion inhibition or retardation
products to protect metal surfaces, especially the thin heat-
exchange tubes where corrosion problems can cost the system owner
the most money. Common yellow metals, such as copper and brass,
usually are protected with tolyltriazole, while steels are protected
with ortho-phosphate.
Biological growth: In order to continuously and
effectively operate a cooling system and establish sound biological
control, first eliminate nutrient sources that may
add material to the system, such as oil leaks,
process fluid leaks and the like.
Second, limit the air-wash effect of the cooling
tower, which naturally adds nutrients and bacteria to the system,
by filtration for removal of suspended solids and the bacteria
that reside on them. Effective filtration can be done either with
centrifugal separators or sand filters. A front- end strainer
also is essential. The uncontrolled multiplication of bacteria,
algae and fungi will result in bio-film formation on heat exchange
surfaces, impairing system efficiency. Stringent regulations exist
to prevent the spread of vapour-borne organisms such as Legionella
L8 from cooling towers and other water systems
Finally, it is critical to provide a consistent
and effective biocide addition to the system. In most cases this
is a chlorine or bromine. Because of regulatory pressures, one
might consider other cooling water biocides like ozone or hydrogen
peroxide. These compounds are very effective biocides when properly
applied and ozone can be used as a stand-alone treatment in most
hvac cooling systems.
In a conventional chemical treatment system, a
service provider or self-administered water maintenance program
consists of adding an oxidizing biocide and a combination scale
and corrosion inhibitor to the water system. One should monitor
chemical treatment to determine the effectiveness of the program.
This will prevent major operating problems in the system.
In a number of cases, additional, specific additives
may be necessary to avoid unique problems presented by operating
water chemistries and specific site issues. A modern pre-packaged
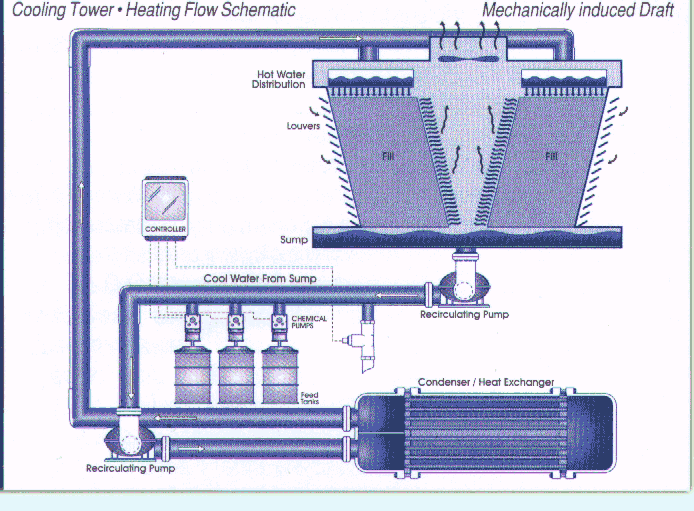
treatment pump and controller system is shown in Figure 1.
Manual method: The traditional water treatment
model includes evaluation, primarily consisting of monthly service
visits that check product inventories, corrosion coupons, probes,
pumps and general equipment maintenance. This type of program
is personnel-dependent and, by its very design, non-continuous.
Automated method: Newer water treatment systems
use electronic monitoring to evaluate the reliability of treatment
delivery and the effectiveness of the treatment system in keeping
the control parameters within specified limits. By using this
type of control, a technician is dispatched only when problems
requiring on-site resolution are encountered.
This labor efficiency increase may offset the
addition-
Limit the air-wash effect of the cooling tower,
which adds nutrients and bacteria to the system, by filtration
for removal of suspended solids
|

